Informação

Before starting the research, developing a protocol that determines which stages the research will cover or which methods will be included in the research is a stage that should be considered. The protocol is the road map in the process of conducting the research. In addition to providing a roadmap for the researcher, developing a protocol also has benefits, such as reducing bias and contributing to accountability. The protocol should include the research topic, the area to be searched (you can determine the databases or languages to be searched at this stage), the keywords to be used in the search, and timing targets. Depending on the type of review, researchers can include the number of articles to be screened or inclusion and exclusion criteria in the protocol.
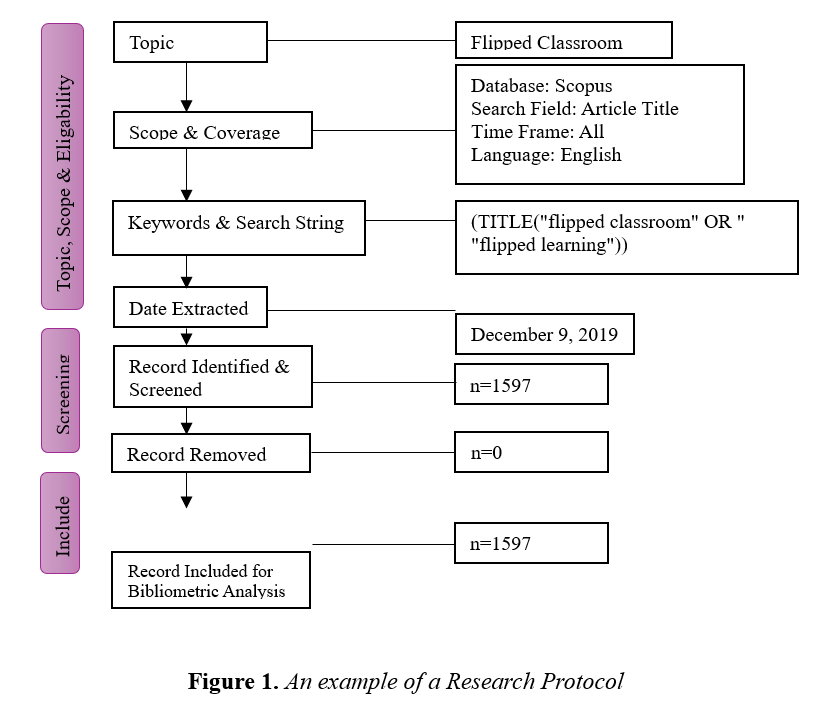
Below is the research protocol that Kushairi and Ahmi (2021) prepared in their bibliometric analysis article titled "Flipped Classroom in the Second Decade of the Millennia: A Bibliometrics Analysis with Lotka's Law ."While the researchers utilized the protocol in the screening and review processes, they also contributed to the reliability of their research by reporting it in the article.
There are several protocol development guidelines for systematic reviews. The Preferred Reporting Items for Systematic Review and Meta-analysis extension for Protocols (PRISMA-P) (Moher et al., 2016) is the most well-known of these guidelines. According to PRISMA-P guidelines, a protocol should include the following components:
“1. Introduction
(a) The rationale for the selection of the selected topic
(b) a clear and understandable research question or research questions, including PICO terms
(a) Inclusion criteria should be set for research and report characteristics (language, publication status). These could be age groups, geographical regions, study designs, or outcome measures. Open inclusion criteria make it easier to identify relevant articles.
(b) All sources of information should be specified with anticipated dates of inclusion (databases to be used, personal contact, use of trial records, sources of gray literature).
(c) Outline the search strategy in at least one database.
(d) A description of how the data will be managed and reviewed throughout the process
(e) The inclusion and exclusion process (e.g., two independent reviewers, etc.) for each part of the review should be described.
(f) The planned data collection method should also describe the data generation process. For example, forms could be included to ensure consistency in data generation.
(g) Any data assumptions or simplifications identified should be listed.
(a) Criteria for which studies will be quantitatively synthesized. Where data are suitable for quantitative synthesis, the planned summary measures, data processing, aggregation methods, and any additional analyses proposed.
(b) Where quantitative analysis is not possible, the type of summary is planned.
The systematic review protocol should be followed at all stages of the review. In addition, changes to the protocol should be tracked and dated (Moher et al., 2016).
In the protocol development process, the importance of developing a protocol that determines which stages the research will cover or which methods will be included in the research is emphasized before starting the research. The protocol serves as a road map for carrying out the research process. In addition to providing a road map for the researcher, developing a protocol also has benefits such as reducing bias and contributing to accountability. A predetermined protocol gives structure and direction to the research process, helps reduce error and bias, ensures that the review is recorded, and may be a requirement for publication.